Clinical Monitoring
Ensuring Study Integrity Throughout Your Programs
With rich experience in clinical trials and a high-quality, professional Clinical research associate (CRA) team, MAVEN provides comprehensive monitoring of all project functions and parameters to ensure the integrity of your program. Our extensive range of services covers phase I-IV clinical trials of drugs and devices development in all major therapeutic areas. Our robust training system ensures every CRA has clinical research experience, regulatory knowledge, and work skills to provide quality services that are consistently trusted and recognized by our partners.

Get Expert CRAs to Rigorously Monitor Clinical Activities at Every Stage
CRA Background

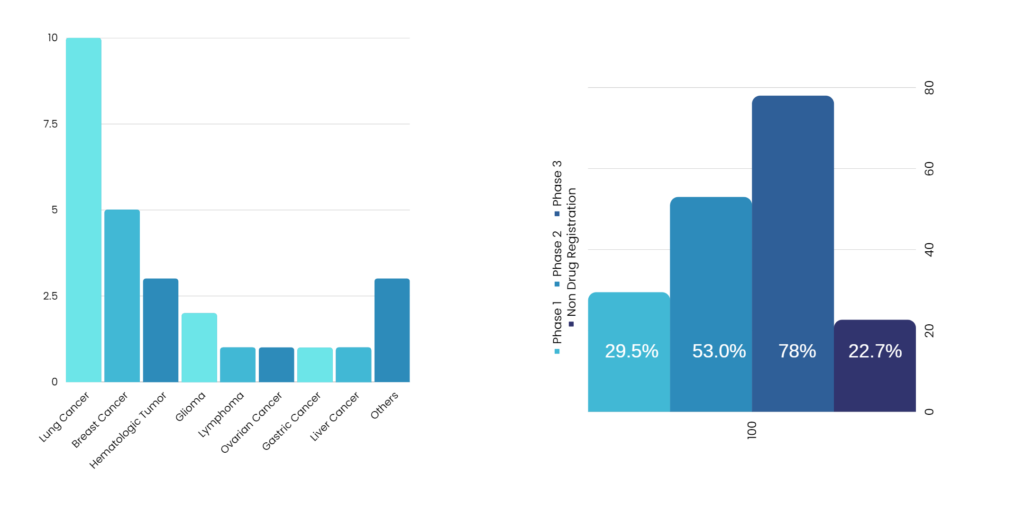
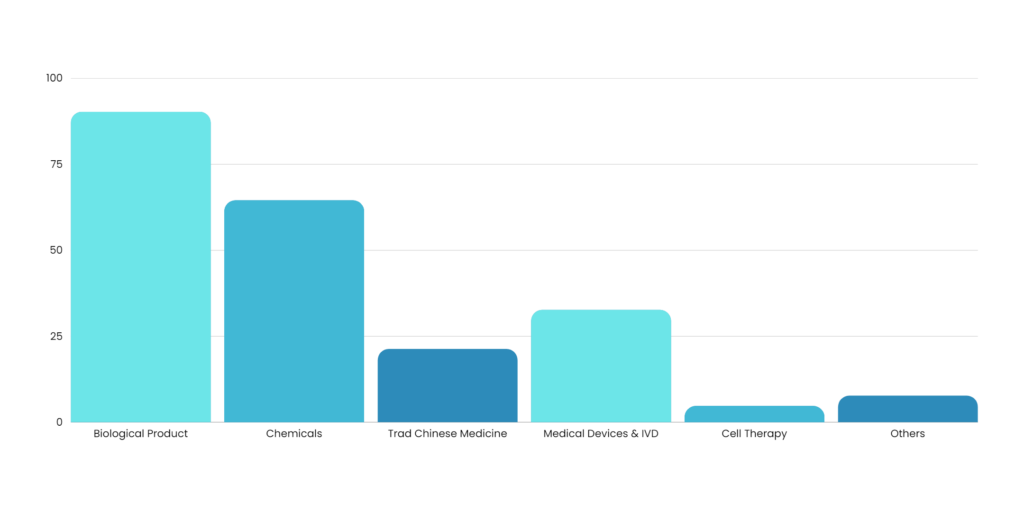

Welcome to Maven, one of the best Regulatory Consultants that is setup by real life working professionals with very rich experience. We at Maven understand the customers pulse and reach their expectations. We are unique and focus on quality is our prime goal. Maven works with any range of company that is looking for quality and in time delivery of services.
Read More




